Start a Pharmaceutical Company in the Netherlands

In this article we will discuss what makes the Netherlands an attractive country to start a pharmaceutical or life sciences company. After which we will walk through the steps to start up a company in the pharmaceutical industry. If you require any advice, do not hesitate to contact us.
Why the Netherlands is a global pharma hub
The Netherlands is one of the global hubs for the pharmaceutical industry. This has a lot to do with its strategic position as a gateway to the European market, but there are many factors at play:
- Advanced public-private partnerships and collaboration.
- Many government incentives to attract pharmaceutical research businesses, such as tax incentives.
- The Netherlands is the home to the most important international medicine regulatory authorities.
- Located at the entry-point to Europe, with Schiphol airport and the port of Rotterdam as its mainports.
- Physical and digital infrastructure that gives the Netherlands an edge over surrounding countries. All within maximum 3 hours driving distance. London, Paris, Brussels and Berlin are within a few hours distance by train or plane.
- Highly-educated, English-speaking population.
- A large and concentrated internal market, while the Netherlands is also one to the top exporters of pharmaceutical products.
- The Netherlands spends relatively large investments on pharmaceutical R&D

Public-private collaboration in pharma and life sciences
The Netherlands has a long history of innovation in the development of medicine. This has lead to a large number of highly-innovative companies in the life science and pharma field. Furthermore, there is a tradition of these companies collaborating with the world-leading universities in the life science field, most importantly Wageningen University. Among many other things, the university's research institute works together with industry on creating innovative ingredients and products which are based on natural raw materials. These public-private collaborations are fundamental to the Dutch way of doing business in the pharma, life sciences and healthcare industries. This becomes physically visible in the many so-called Science Parks the Netherlands has. Often these are clusters of businesses and public initiatives. As an example, the Leiden Bio Science Park focuses on the biotechnology sector and ranks among the top science parks in the world. In short:
- Over 300 public-private partnership in pharma and life sciences. Examples are RegMed and Oncode
- 3000+ R&D companies in the life sciences
- 8 University Medical Centers
- 13 universities involved in life sciences research and education
A pharma-friendly business climate
Furthermore, the Netherlands lures pharmaceutical companies to the Netherlands using tax incentives. Such as a lower corporate tax rate for highly-innovative businesses. But also an income tax break to attract highly-skilled workers from abroad, called the 30 percent ruling.
The Netherlands is often used as a gateway, not so much as a production country. In the pharmaceutical industry, this is a different story. Research show that the Netherlands is booming in terms of the production of pharmaceutical products. For example, basically all big pharmaceutical companies (Johnson & Johnson, MSD/Merck, Gilead and others) are building or expanding production facilities in the Netherlands. Furthermore, it is not only production that is expanding, also the development of the medicines that the big pharma companies produce, seems to be moved increasingly to the Netherlands. Biotech companies such as Pharming and Europe's largest Biotech company, Danish Genmab, are investing heavily.
There is not one clear reason why the Netherlands has become a pharmaceutical powerhouse. And what is different now compared to few years ago. The Netherlands has had a long tradition of innovation in the life sciences. And it houses many pharmaceutical companies, research facilities, public initiatives, funding schemes and tax incentives. This has lead to many pharmaceutical companies and organizations setting up in the Netherlands. Many experts point at the move of the European Medicines Authority (EMA) from London to Amsterdam after Brexit, as a defining moment. Following this, many pharmaceutical and healthcare businesses and institutions followed suit and set up an office or production facility in the Netherlands. On top of this, the US Food and Drug Administration (FDA) has its European offices in the Netherlands (embedded in the EMA).

Growth market internally and externally
The Netherlands has a large internal market for pharmaceutical products. Just like in rest of Europe, this is a growth market mostly due to an ageing population. And although the import of pharmaceutical products has increased, the export from the EU to the rest of the world is increasing even more. Also here, the Dutch pharmaceutical industry punches above its weight being the 4th largest pharmaceutical exporter in the EU (2019).

Tax advantages for pharmaceutical companies in the Netherlands
There are several tax advantages which may apply to your pharmaceutical or life sciences business in the Netherlands. These are designed to stimulate innovation and production in the Netherlands and to attract talented highly-skilled individuals.
Innovation Box
The first one is the so-called Innovation Box. This is a relevant incentive for pharma and research businesses since it stimulates innovative and high-tech research. All profits you subsequently make from this research and innovation falls into the "Box". The corporate income tax in the Innovation Box is currently 9 percent. That is a large cut compared to the regular corporate income tax rates below.
| Profit | 2023 | 2024 |
|---|---|---|
| SME tariff | 19% (up to €200.000) | 19% (up to €200.000) |
| Standard tariff | 25,8% (profits exceeding €200.000) | 25,8% (profits exceeding €200.000) |
| Innovation Box | 9% on profits derived from qualifying innovative activities | 9% on profits derived from qualifying innovative activities |
There are some requirements you should meet to successfully apply for the Innovation Box. It is quite an administrative process, but if you are expecting to conduct serious and innovative research with large profits as a result, the application can definitely be worth it. Contact us for more information or to bring you in touch with a Innovation Box tax specialist.
30 percent facility
The objective of the 30%-ruling is to bring highly-skilled workers to the Netherlands. To successfully apply for this facility, the employee needs to come from outside of the Netherlands. The worker should meet the following requirements:
- You have an employment relationship;
- You are recruited from another country by your first employer in the Netherlands, or you are on assignment in the Netherlands from another country;
- Of the 2 years before your 1st working day in the Netherlands, you lived outside the Netherlands for more than 16 months, at a distance of more than 150 kilometers from the Dutch border;
- You have specific expertise that is not or is only barely available on the Dutch employment market;
- You have a valid decision from the tax authorities.
If the conditions are met the worker does not have to pay tax over a maximum of 30 percent of his or her wages. This is especially lucrative for those with a higher pay, because of the progressive income tax system (see overview below).
| Belgium | Denmark | Ireland | Netherlands | Finland | Sweden | Denmark | Estonia | |
|---|---|---|---|---|---|---|---|---|
| Local office lease requirement | Public limited company (société anonyme/naamloze vennootschap) Service agreement or lease agreement for registered office or property. Limited company (société privée à responsabilité limitée/besloten vennootschap) Service agreement or lease agreement for registered office or property. Belgian branch office of a foreign company A Belgian branch office must have a physical existence in Belgium (ie, an office in which the foreign company carries out its activities in Belgium). Third-party service providers can provide a Belgian branch office with such local office. | Limited liability company (Kapitalselskab) Limited companies must have a registered office in Denmark, but it does not have to be either owned or leased by the company. | Private company limited by shares (LTD) Every company is required to have in Ireland: a registered office address , being the official address where all formal legal documents are sent or a business or trading office address. Both the registered office address and business/trading office address can be the same address and may be provided by a third-party services provider. | |||||
| Sufficiency of virtual office | Limited company (société à responsabilité limitée/besloten vennootschap) The registered address of the public limited company can be set with the accountant or can be a postbox office with a third-service provider. Belgian branch office of a foreign company See local office lease requirement. | An address is needed, but here are no requirements as to the presence of directors or employees on that address. | Private company limited by shares (LTD) Insufficient – a physical address must be specified on incorporation documentation. External company Insufficient – a physical address must be specified on registration documentation. | An address is needed, but there are no requirements as to the presence of directors or employees at that address. | ||||
WSBO - tax cut on loan costs for R&D projects
If you are an innovative business in pharma and life sciences, you might want to look into the WSBO. This is effectively a R&D tax relief through a payroll withholding tax credit. You need to be planning on developing a project, production process or software. And your business should be the one in charge of solving technical issues itself. This cannot be outsourced if you want to make use of the WSBO. You can find a list of requirements on the website of the Dutch government.
Find more tax incentives and advantages in our country guide. We also recommend you to read this article on the various R&D-incentives for businesses across Europe.
Legal entity and structure for a Dutch pharmaceutical business
Because pharmaceutical businesses usually carry a relatively large amount of risk, the BV is the preferred legal entity for Dutch pharma businesses. This BV is a limited liability company comparable to the UK Limited company (Ltd.) and the German GmbH. In the BV your private assets are protected against losses or bankruptcy of the company.
The second reason to choose the BV for a business in the pharmaceutical or healthcare business, is because many tax advantages only work for a BV and not for example a partnership. This has to do with the fact that a BV is its own legal entity, where a sole proprietorship or partnership is considered to be fiscally united with its owners.
A third reason is the fact that a BV is seen as a more serious legal entity. Lots of large international firms have a "BV" behind their name, which improves your reputation and generates trust among customers and suppliers.
Holding BV structure
A final and very important reason the BV makes sense, is how you can use the BV as a tool to structure and expand your business. The Dutch BV holding regime has been of the most popular company structures in the world. Many businesses set up a holding BV to manage their subsidiaries throughout Europe and the world. This has to do with the fact that the Netherlands has a large amount of tax treaties. A crucial part of this holding regime is the existence of the so-called participation exemption (or in Dutch: Deelnemingsvrijstelling). The main advantage of the Dutch participation exemption is that it prevents profits that have been taxed in a daughter company are taxed again in the mother company (the holding BV). This can be used within Netherlands but usually also with subsidiaries abroad.
A related advantage is that the distribution of dividend by a Dutch company to a company resident in another country is often exempt from withholding dividend tax. This applies if a foreign company owns at least 10 or 25 percent (depending on the tax treaty) of a Dutch BV.
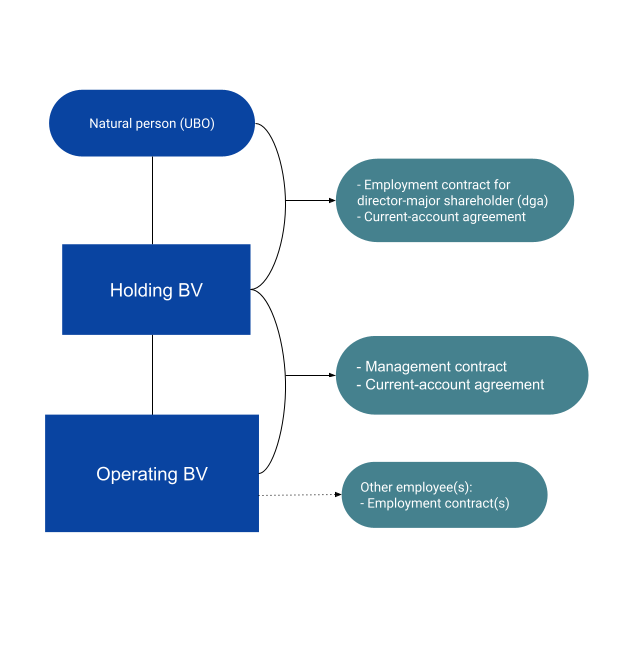
If you are setting up a pharmaceutical company in the Netherlands we advise you to set up one holding BV per shareholder where every shareholder can accumulate profits. This holding BV will hold shares in an operating BV where the company's actual activity takes place. For example the production, distribution and marketing activity take place in the operating BV. Any profits in this Operating company are subsequently distributed to the holding BV('s) of the shareholders. Let's say that a completely new product or medicine will be developed which has nothing to do with the other one. In that case it can be beneficial to set up a second operating BV. A few advantages:
- In case of bankruptcy of one BV, the others are not affected even if they belong to the same group.
- The holding structure makes it easy for founders to establish side businesses where all shareholders or just a few are involved. The structure does not need to be changed if a new activity is started.
- A sale shares in an operating BV (for example if one of the partners/shareholders wants to leave the company) can be distributed tax-free to that shareholder's holding BV.
Find out much more about the Dutch holding regime in our comprehensive guide on starting a business in the Netherlands. For more on the process of registering a BV company, you should check out our timeline.

Permits for pharmaceutical companies in the Netherlands
The pharmaceutical industry consists of a wide range of business types. The core activities are business specialized in discovering new drugs and treatments, developing those medicines and eventually produce them. Besides those primary pharmaceutical companies, there are many related businesses. For example, wholesale businesses marketing and selling the medicines, but also companies specialized in distribution, packaging or preparation of pharmaceuticals. Most of these business need so some of permit to operate.
Once your company is officially registered, you should take look at which permits you need to operate your pharmaceutical business.
Wholesale permit
If your pharma business is involved in the selling and distributing from within the EEA (EU countries plus Norway, Iceland and Liechtenstein), you will need a wholesale license in the Netherlands. This license is called Groothandelsvergunning or GDP in Dutch. There is a registration fee of +/- €1700 and an annual fee of a similar amount.
Manufacturer's permit
This is relevant for those businesses that prepare medicine. But also for those who import medicines from outside the EEA. Packaging and labeling are seen as 'preparing' and are thus also covered by this manufacturer's permit. Like the wholesale permit, the manufacturer's permit is processed by Farmatec. The process can be started by filling out a form. The fees are higher than the wholesale permit's.
Broker's license
As a broker you are not involved with the physical shipping and distribution of the medicines (wholesale). However, under Dutch law you will need a broker's license. More information on the broker's license can be found on the Farmatec website.
Producing or selling
The Active pharmaceutical ingredient (API), is the biologically active component of a medicine. Those who sell or manufacture API's need to be registered with Farmatec. In addition they should comply with the EU's Good Manufacturing and Distribution Practices (GDP)
Further permits and licenses
- If you produce or deal in API's for veterinary medicine, you should normally register with Farmatec
- Your business is involved in blood products you will probably need a permit. This applies if you are importing into or exporting from outside the EEA. But also if you are delivering blood products to another EEA country that is not the Netherlands.
- Organ banks need approval.
- Donor test labs require a permit.
Logistics and pharmaceuticals
Usually the physical distribution of medicine or other pharmaceutical products is outsourced to specialized distribution and transportation companies. You can check this directory if you are looking for a specialized company. For example this company can take care of all your logistics.
Employment contract in the pharmaceutical industry in the Netherlands
A standard employment contract is sufficient to employ someone in a pharma business. You should be aware of the collective labour agreements that apply in many of the pharmaceutical sectors. For example jobs in the sector "pharmaceutical trade" are regulated by a specific collective labour agreement. This means for example that a minimum wage for this sector applies and several other agreements to protect workers. In practice, your employment agreement cannot be in conflict with this collective labour agreement.
Certain processes of pharmaceutical businesses can be extremely privacy-sensitive. It is therefore that GDPR compliance should be set up well and be kept up-to-date even more than in most other industries. If you are dealing directly with consumers, for example selling medicine through a shop or online, these patients' privacy is of the utmost importance. We have created a GDPR compliance check that filters out what legal documents you should have in order to become fully GDPR compliant.
Introducing new medicines to the Dutch market
Do you have a pharma company in the Netherlands and you want to bring a new medicine to the Dutch and European market? There are a few procedures you should follow and a few other things you should be aware of. To be clear, this concerns applications for medicines for human use.
There are several procedures to put a new medicine on the Dutch or European market. Therefore, you need to establish what medicine you are introducing and where you want to introduce and distribute it:
- National procedure - only valid for medicine you will bring to the Dutch market.
- Decentralized procedure - should be used to obtain a marketing authorisation in several EU member states. This is relevant when you do not yet have a marketing authorisation in any country.
- Centralized procedure - instead of taking the national procedure, the centralized procedure will make your medicine available to all European residents. Applications are processed by the EMA in Amsterdam.
- Mutual recognition procedure - in this case the medicine has already been accepted in one EU member state (for example in the Netherlands after following the "National procedure". This procedure requests mutual recognition for that approval in an other member state.
You will need to have approval from the Medicines Evaluation Board (MEB) for your new drug. On the board's website you can find a step-by-step overview of how to submit your application. This application will contain the most important details about the new medicine. Among other things, the substances in the medicine and risks involved in using it. But at this stage you also already submit the label and packaging text. There MEB sets strict requirements to the packaging. For example, every package needs to contain braille. Find all requirements on this page.

Finding capital for a pharmaceutical company in the Netherlands
With an ageing population and the effects of a worldwide pandemic more apparent than ever, investors are lining up for investments in pharmaceutical, healthcare and life science businesses. Still, it may be hard to navigate the Dutch investment world if you are not familiar with it.
Regional innovation push
As said, there are many government or semi-public initiatives in the life sciences and health sector. Many of the funding efforts from the government are done through regional investment agencies (ROM's). There are eight of those in the Netherlands, some more specialized in life sciences than others. Arguably the most relevant one is the ROM of the province of Noord-Brabant, int he south of the Netherlands. This region is seen as the innovative heartland of the Netherlands. The Eindhoven region is specialized in medtech, and north-east Brabant focuses on pharma.

Other regions are picking up and investment is seen across the entire country. On the other side of the country, the development agency for the north of the Netherlands has started the Pharma Connect Capital (PCC) investment fund. This fund is specialized in, among other things, very early-stage medicine development. And the most populous region (Den Haag, Rotterdam area) should be mentioned as well. This region is home to three of the top universities (Delft, Rotterdam and Leiden) within 30 minutes driving distance and a dense network of Science/Bio Parks, production and R&D facilities. Their regional investment fund, InnovationQuarter brands itself as the go-to region for life science businesses.
Private funds
There are numerous VC funds and other investors interested in investing in life science and pharmaceutical products. Most of them specialize in a particular part of the process, from early-phase development til distribution and logistics. Finding the right investor can be a real challenge. Especially if you are new to the Dutch business world.
At NordicHQ, we specialize in navigating foreign entrepreneurs and established companies through the Dutch business landscape. Whether it concerns company formation, tax and legal compliance or industry-specific rules and regulations. Moreover, using our network we can connect you to relevant specialists, investors and discover new opportunities. Read much more about this in our funding guide for international companies in the Netherlands.

